Latest Research News
Rapid removal of emerging endocrine disruptors in wastewater using high-performance single-atom catalysts
- Date : 24-08-13
- Views : 312
- Developing high-performance single-atom catalysts through chemical-free dry processes and computational science.
- Rapid removal of bisphenol, an endocrine disruptor, in water treatment process
Bisphenols are widely used as the main raw material for plastics such as receipts, water bottles, water containers, and vinyl due to their heat-resistant and mechanochemical properties. Among bisphenols, bisphenol A (BPA) that we often refer to as an "endocrine-disrupting chemicals" has been linked to adverse effects on reproduction, development, intelligence, and various metabolic diseases. Bisphenol F (BPF), a recently developed alternative to BPA Bisphenol A has also been reported in the literature to cause neurological disruption and various health risks.
Dr. Jong Min Kim of the Materials Architecturing Research Center, Dr. Sang Soo Han of the Computational Science Research Center, Dr. Sang Hoon Kim of the Extreme Materials Research Center at Korea Institute of Science and Technology (KIST), and Professor Byeong-Kwon Ju of the School of Electrical Engineering at Korea University have fabricated high-performance cobalt single-atom catalysts through a chemical-free and environmentally friendly dry-based arc plasma deposition process. The team applied it to an electro-Fenton process based on electrochemical hydrogen peroxide synthesis to remove harmful bisphenols from aqueous solutions in a short time.
The arc plasma process vaporizes metals or ceramics with repeated pulsed voltages in a vacuum, depositing them as a thin film on the surface of the substrates, and the number of pulses can be controlled to create a deposited layer with the desired thickness or properties. The cobalt single-atom catalyst fabricated by the arc plasma process exhibited the world's highest metal single-atom loading (2.24 wt%) compared to previously reported single-atom loading of dry processes (around 1 wt%). The coordination structure and active sites of the prepared Co single-atom catalyst were characterized by various material analyses including computational science, and electrochemical measurements confirmed that it is an excellent single-atom catalyst for electrochemical hydrogen peroxide production.
The researchers applied the Co single-atom catalyst as an electrode to supply hydrogen peroxide in real time in the electro-Fenton water treatment process, and found that it could rapidly degrade 100% of BPF at a targeted concentration of 20 ppm in aqueous solution within 5 minutes. Through repeated experiments and wastewater treatment tests, the stability of the catalyst and the removal of bisphenol compounds were verified, and based on this, it is expected to be applied to the removal of emerging pollutants in wastewater treatment plants in large cities or specific industrial wastewater treatment facilities.
"This achievement is significant in that we have produced high-performance single-atom catalysts in a dry process that does not use harmful chemicals and applied them to the water treatment field," said Dr. Jong Min Kim of KIST, while Dr. Sang Hoon Kim of KIST said, "Research on the production of metal nanoparticles by arc plasma deposition is widely known, but this is the first study to show that single-atom deposition is possible.“
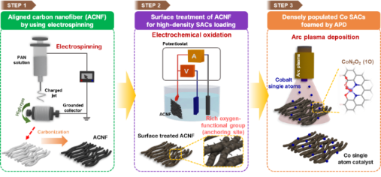
[Figure 1] Schematic illustration of the synthetic process of Co single-atom catalyst using arc plasma deposition.
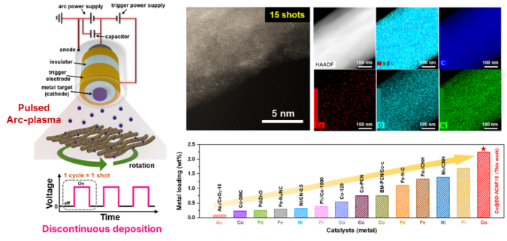
[Figure 2] Images of a Co single-atom catalyst prepared using arc plasma deposition (APD) and comparison of loading amount of single atoms using a conventional dry process.
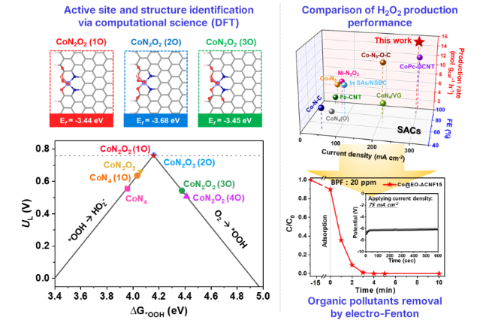
[Figure 3] Identification of the active sites of electrochemical hydrogen peroxide production reaction on Co single-atom catalyst using computational science and its application to the rapid removal of bisphenol F (BPF), an organic pollutant, using electro-Fenton.

[Figure 4] High-performance Co single-atom catalyst supported on carbon nanofibers developed by KIST researchers through a dry-based arc plasma deposition process.
###
KIST was established in 1966 as the first government-funded research institute in Korea. KIST now strives to solve national and social challenges and secure growth engines through leading and innovative research. For more information, please visit KIST’s website at https://eng.kist.re.kr/
The research was supported by the Ministry of Science and ICT (Minister Lee Jong-ho) through the KIST Major Project and Nanomaterial Technology Development Project (NRF-2022M3H4A7046278) and the Ministry of Environment. This research was published online on July 5 in the SCI journal Carbon Energy (IF: 19.5, JCR: 3.8%).